Source: Syngenta
Soil potential hydrogen (pH) is the amount of acidity or alkalinity of soil. It is measured on a scale of 1 to 14, where 7 is neutral. Soil pH is a measurement of the hydrogen ion activity in the soil solution, which is influenced by the type and amount of clay and organic matter content in the soil.
Soil tests may show a buffer pH result, which indicates the amount of agricultural lime required to neutralize the hydrogen ions from the soil. “Buffer” refers to the ability to release acidity ions into the soil; for example, high clay soils are highly “buffered” and require more lime to raise pH to a certain level than sandy soils.
How pH Affects Soil Nutrients
The availability of some plant nutrients is affected by soil pH. Field crops perform best at a soil pH between 6.0 and 6.8, depending on the crop. In many areas of the Corn Belt, soil pH can decrease or become more acidic due to factors such as:
- Nutrient removal by crops
- Leaching of basic nutrients (cations in the soil)
- Using ammonia-based nitrogen fertilizers
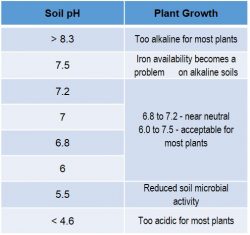
Source: Colorado State University – CMG Garden Notes #222
Correcting Soil pH
Adding liming materials can raise soil pH levels for ideal crop production where nutrients are more available to the plant, as well as create a healthy environment for critical soil microbes.
The University of Nebraska-Lincoln recommends the following adjustments of lime rates for different depths of incorporation:
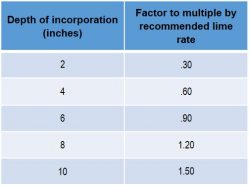
For more recommendations on pH management, speak with your local NK retailer.
